Different Atoms of the Same Element May Have Different
Terms in this set 92 Different atoms of a particular element can have different atomic mass numbers. Quantum mechanics problem Please provide a well explained and correct solution for the following.

Are Two Atoms Of The Same Element Identical Science Questions With Surprising Answers
These various atoms are called.
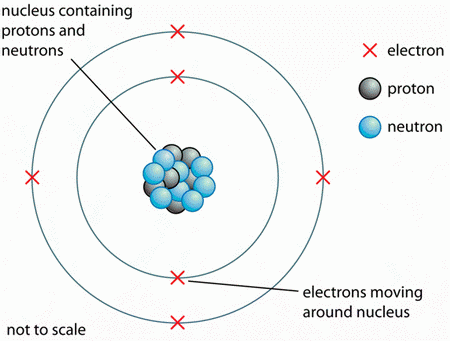
. Protons Neutrons Isotopes Nucleon Number mass number Proton Number atomic number. 5 Do all oxygen atoms have the same properties. The nucleus in the center is.
The size of a anion is generally _____larger or smaller than the parent atom 4 Two atoms that have different Q. 6 How is oxygen different from all other elements. We refer to the atoms of the same element with different numbers of neutrons as isotopes.
4 How are atoms and molecules related. 7 Are oxygen atoms are different from the atoms of other elements. 8 Are all atoms the same.
An element is a substance that is made entirely from one type of atom. 1Circle the letter of each sentence that is true about Daltons Atomic Theory aAll elements are composed of tiny indiv. For example a diamond coal and soot all are.
The atomic mass of individual atoms of an element may vary. Atoms of the same element that are arranged in different molecular formations are called allotropes. The main difference is elements are made of atoms.
Therefore C is correct. Atoms of the same element may differ in their number. This option is correct statement.
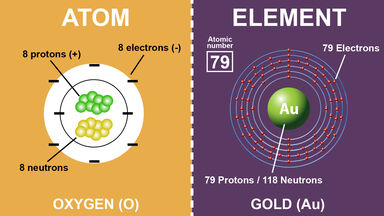
The identity of an element is determined by the number of protons each of its nuclei contains. Different atoms of the same element Different Atoms of the Same Element GCSE Keywords. For example the atomic number of carbon and nitrogen is 6 and 7 respectively.
What is the m. In their center atoms have a closely packed nucleus. Explore examples of elements and atoms.
Learn other differences between atoms and elements by dissecting these two terms. 1 How Are Atoms Different From Molecules. Atoms of the same element can differ in their mass which is the case if the number of neutrons in the atoms is not the same.
2 What is the most important difference between atom and molecule. Yes isotopes of an element do have different atomic mass numbers. Protons and electrons are oppositely charged.
Isotopes are different atoms of the same element with the same atomic number but different mass numbers. Some ratios of protons to neutrons are more stable than other ratios. Many people might think atoms and elements are the same.
These atoms with different mass are called isotopes. Atoms with same number of neutrons but different atomic number 3. Isobars are the atoms having the same mass number but different atomic number.
For example in hydrogen theres 3 Isotopes for hydrogen. Why or why not. 7 How do the molecules of a compound differ from the.
Isotopes are defined as. Its different atoms from a same element that has the same number of protons but different number of neutrons. Again proton for the same element is never changed even if theyre different Isotopes.
Yes these are called isotopes. The number of protons then determine which element the atom is. 6 What is the main difference between an atom and an ion.
Atoms in a ________ dissociate in water. 9 What do atoms of the same element have in common. So E is correct.
The other elements have been produced artificially or as a result of nuclear reactions. Electrons are HUGE compared to protons. 5 Why are atoms not molecules.
Potassium aluminum sulfate dodecahydrate KAl SO 4 2 12H 2 O or alum is used as an aftershave. Can atoms of two different elements have the same number of protons. A chemical bond between atoms that share electrons is called ________ bond.
For example the element hydrogen is made from atoms containing just one. Hence the statement which is completely accurate is atoms of the same element can have the different number of neutrons. Atoms of the same element that have the same number of neutrons.
Can a single atom be an element. Atoms are the simplest unit of a matter. Carbon-14 an isotope of carbon has a mass number of 14 which is same as that of nitrogen and.
Write two truths and a lie about the subatomic particles. 11 What does all element have in common. The same chemical element can have a different number of neutrons and still be the same element.
The number of neutrons have no effect on the chemical properties but do affect the nuclear properties. Answered over 90d ago. Learn more about the atom here.
However atoms and elements do have a few differences when you start breaking it down. The atomic mass of a single carbon atom is equal to exactly 12011 amu. For example U235 with a ratio of 92 protons to 143.
Atoms with same mass number but different atomic number Select the correct answer using the code given below. Atoms of the same element can have the different number of neutrons-atoms of the same element can have the different number of neutrons known as isotopes. While the particular isotope involved does not affect how an atom will react chemically it does determine how the atom will behave in nuclear reactions.
3 How are molecules different from atoms quizlet. Allotropes may have different physical appearances and different characteristics such as electrical conductivity. The number of protons determine the chemical properties of an atom.
The difference in mass arises due to the atoms containing a different number of neutrons for the same number of protons. Atoms of the same element that have different number of protons. 10 Do all atoms of the same element have the same atomic number.
Therefore to be precise atoms are the smallest part or amounts of elements. Choose any two elements. Atoms of the same element that have different number of neutrons.
This is the primary difference between an atom and element. Isotopes are atoms of the same element that have a different number of neutrons. Answered over 90d ago.
Isotopes are when atoms of the same element have a different mass number due to a different number of neutrons. Atoms with same atomic number but different atomic mass 2. Atoms that have the same atomic number but different atomic masses are called isotopes.
Atoms of the same element that have different number of electrons.
Difference Between Atoms And Elements With Examples

Understanding Atoms Elements And Compounds Lesson And Worksheets
No comments for "Different Atoms of the Same Element May Have Different"
Post a Comment