Why Is Calcium More Reactive Than Magnesium
First week only 499. The reactivity series allows us to predict how metals will react.

Why Is Calcium More Reactive Than Magnesium Lisbdnet Com
Valence electrons are on the outermost occupied energy level in the atom and they cause the atom to react and thus the further the Valence electrons are from the nucleus in an atom the more reactive is the element.
. And why is calcium less reactive than potassium. Weve got the study and writing resources you need for your assignments. Start your trial now.
These metals are less reactive than the neighboring alkali metal. See full answer below. Calcium is more reactive.
Rusting is an oxidation reaction. The elements of this group is known as alkaline earth metals. Solution for Why is calcium more reactive than magnesium in general.
A more reactive metal will displace a less reactive metal from a compound. Because of its larger size calcium has one more. Which metal is more reactive than magnesium.
Is calcium or magnesium more reactive. Calcium is more reactive than magnesium because it is larger than a magnesium atom because it has one more energy level. Calcium is more reactive than magnesium because the calcium atom is larger than.
Magnesium has a lower activity than sodium calcium has a lower activity than potassium and so on. Ca and Mg are present in group-2 of the peridic table. As we move down the column these metals become more active.
In the Periodic Table Calcium is directly below Magnesium which means that a calcium atom is larger than a magnesium atom. By signing up youll get thousands of step-by-step solutions to your. Ionization energy decreases when we go down in the group.
They donates electron to form metal ions and form ionic bonds combined to the non-metal ions The element can easily donates electron is more active. Magnesium has a higher activity than beryllium calcium has a. Calcium is more reactive than magnesium because the calcium atom is larger than the magnesium atom.
So this entails that my calcium is more reactive then Magnuson. Why is magnesium less reactive than sodium. See answer 1 Best Answer.
Answer 1 of 3. The alkaline-earth metals tend to lose two electrons to form M 2 ions Be 2 Mg 2 Ca 2 and so on. Calcium is more reactive than magnesium because it is larger than a magnesium atom because it has one more energy level.
Explain why calcium is generally more reactive than magnesium. These metals are less reactive than their alkali metal neighbors. In this exercise we have to answer why is calcium generally more reactive than magnesium.
Calcium has less ionization energy than magnesium and thats why calcium is more reactive than magnesium. Since the electrons are farther from the nuclear use in calcium ion than in magnesium ion due to the innate large size of calcium it follows that it can be the electrons where the outermost electrons of calcium can be easily removed from the atom itself. Calcium and magnesium are in the same group.
Is Calcium More Reactive Than Zinc Quora
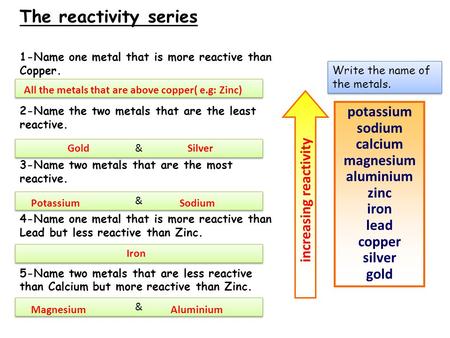
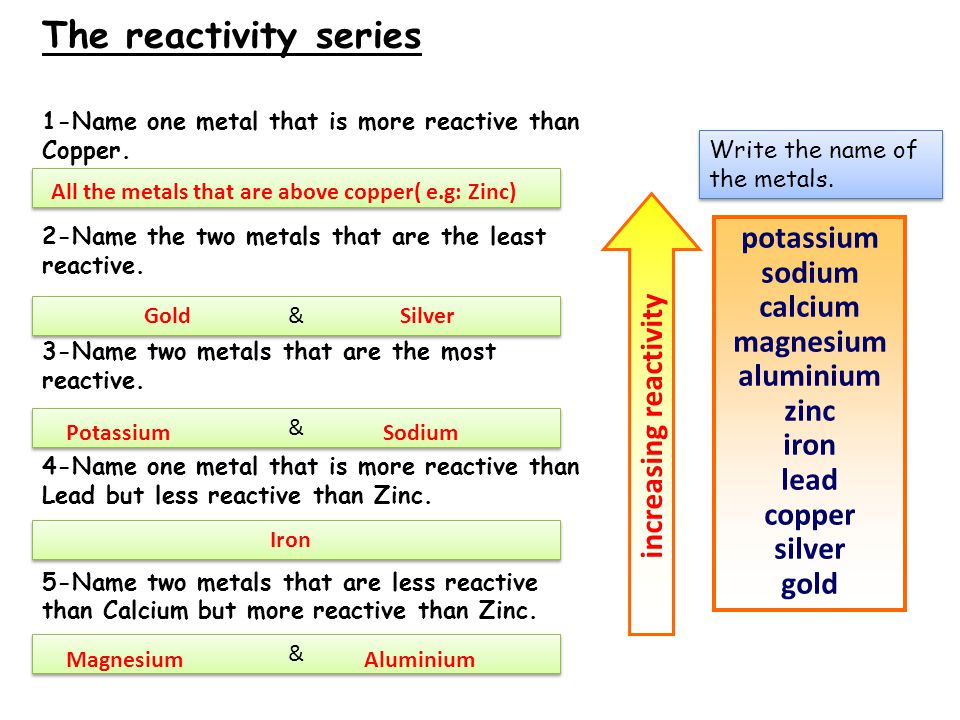
Reactivity Of Metals D Isplacement R Eactions Magnesium Sulphate Zinc Sulphate Iron Sulphate Copper Sulphate Magnesium Zinc Iron Copper Ppt Download

Which Is More Reactive Na Or Ca Quora

Why Is Calcium Generally More Reactive Than Magnesium Study Com
What Is The Reactivity Order Of Na K Li And Mg With Water Quora

Ppt Section 7 7 Group Trends For The Active Metals Powerpoint Presentation Id 5519982

Why Is Calcium More Reactive Than Magnesium Lisbdnet Com
No comments for "Why Is Calcium More Reactive Than Magnesium"
Post a Comment